Answer:
8.03L
Step-by-step explanation:
Given parameters:
Initial volume, V₁ = 8.7L
Initial temperature T₁ = 50°C , in Kelvin gives 50+273 = 323K
Final temperature T₂ = 25°C, in Kelvin gives 25 + 273 = 298K
Unknown:
Final volume V₂ = ?
Solution:
Since we are dealing with volume and temperature relationships under constant pressure, we simply apply the Charles's law.
Charles's law states that "the volume of a given mass of gas is directly proportional to the temperature provided the pressure remains constant".
Mathematically, we have;
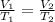
where V andV T are volume and temperature, 1 and 2 are initial and final states.
Input the parameters and solve for V₂;
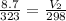
V₂ = 8.03L