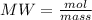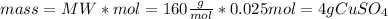Answer:
A) 4 g
Step-by-step explanation:
Data:
V =100 mL = 0.1 L
M = 0.25M
g = ?
Molarity
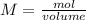
The moles are cleared from this equation:
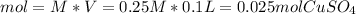
Through the molecular weight, the grams of CuSO4 are calculated, knowing that the molecular weight of CuSO4 is 160 g/mol.