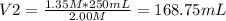Answer:
a. C. volumetric flask
b. B. Measure out x mL using a volumetric pipet
c. 168.75mL
d. B. Add the correct amount of stock solution then fill to the 250 mL mark with water.
Step-by-step explanation:
- The volumetric flask is mainly used in analytical chemistry to contain standard solutions or to prepare dilutions of a certain concentration of a reagent.
- The volumetric pipette is used to deliver a given volume with greater precision and accuracy.
- To make a dilution, the formula C1*V1 = C2*V2 is used where C is the concentration of the solution, in this case of molarity. V is the volume of the solution in ml and terms 1 and 2 correspond to the concentrated and diluted solutions respectively.