Answer:
Gold
Step-by-step explanation:
We have
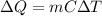
where, $Q$ is heat supplied, $m$ is mass, $C$ is specific heat capacity and $\Delta T$ is rise in temperature.
Thus, higher the $C$, greater is the heat required to hear it to a certain temperature.
Gold has the least Specific Heat capacity among the options, hence will require the least amount of heat to raise the temperature.