The Volume of the balloon indoors in increased by 3.8 L.
Step-by-step explanation:
As per the Charles law, at constant pressure the volume of the gas is in direct proportion with the temperature measured in Kelvin. As temperature increases, the volume expands that is increases and vice-versa.
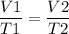
T1 = -35° C + 273 K = 238 K
T2 = 25° C + 273 K = 298 K
V1 and T1 are the Volume and the temperature of the balloon inflated outdoors.
V2 and T2 are the Volume and the temperature of the balloon inflated indoors.
V2 can be found by rearranging the above equation as,
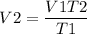
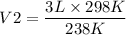
= 3.8 L
So the Volume of the balloon indoors in increased by 3.8 L.