55.36 is the mass of Calcium Bromide is needed to make 0.500 L of 0.554 M solution.
Step-by-step explanation:
Data given:
mass of Ca
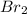 = ?
= ?
molarity of the calcium bromide solution = 0.554 M
Volume of the calcium bromide solution= 0.5 L
First the moles of calcium bromide is calculated as:
molarity =
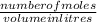
number of moles = 0.554 x 0.5
number of moles = 0.277 moles of calcium bromide
atomic mass of calcium bromide = 199.89 grams/mole
mass = atomic mass x number of moles
mass of calcium bromide = 199.89 x 0.277
= 55.36 grams
mass of calcium bromide required is 55.36 grams to make solution of 0.554 M in a solution of 0.5 litres.