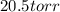The question is incomplete. The complete question is:
15L tank of gas is contained at a high pressure of
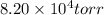 . The tank is opened and the gas expands into an empty chamber with a volume of
. The tank is opened and the gas expands into an empty chamber with a volume of
![6* 10^4 L. What is the final pressure if the temperature remains constant.</p><p><strong>Answer: 20.5 torr</strong></p><p><strong>Explanation:</strong></p><p>To calculate the new pressure, we use the equation given by <strong>Boyle's law. </strong>This law states that pressure is directly proportional to the volume of the gas at constant temperature. </p><p>The equation given by this law is:</p><p>[tex]P_1V_1=P_2V_2]()
where,
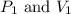 are initial pressure and volume.
are initial pressure and volume.
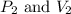 are final pressure and volume.
are final pressure and volume.
We are given:
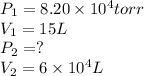
Putting values in above equation, we get:
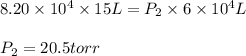
Thus new pressure will be