Complete Question
The complete Question is shown on the first and second uploaded image
Answer:
The underlined words are the answers
Part A
Moving from boron to carbon, the intensity of the bulb Increases because Z increases from 5 to 6 , The thickness of the frosting stays the same because the core electron configuration is the same for both atoms
Part B
Moving from boron to aluminum the intensity of the bulb Increases because Z increases from 5 to 13 . The thickness of the frosting also increases because Al has the core configuration of Ne, while B has the core configuration of He
Step-by-step explanation:
Here Z denotes the atomic number
Ne denoted the element called Neon and its electronic configuration is
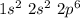
He denoted the element called Helium and its electronic configuration is
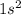
B denoted the element called Boron and its electronic configuration is
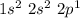
Looking at its electronic configuration we can see that the core is He
I,e
![[He]\ 2s^2 2p^1](https://img.qammunity.org/2021/formulas/physics/college/zjm5o46ytm17hqphoqcxr8ahy341bv8j5z.png)
Al denoted the element called Aluminium and its electronic configuration is
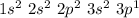
Looking at its electronic configuration we can see that the core is Ne
I,e
![[Ne]\ 3s^2 \ 3p^1](https://img.qammunity.org/2021/formulas/physics/college/h69fdlsbtkpecya9vh2xyccdrz2tm25o79.png)