Answer:
The meaning f mathematical term is given below
A - This is defined as the absorbance of that solution which have all Bound hemoglobin imidazole comlex.
so according to this [Hb.Fe3+] = 0
[Hb.Fe3+.Im]= 100
concentration can be calculated by lambert beer law
A=
 Cl
Cl
where A = absorbance
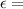 molar absorbance coefficient
molar absorbance coefficient
l = path length
A0 - This is defined as the absorbance of compeletely unbound hemoglobin . this is for non complex with imidazole.
[Hb.Fe3+.Im]= 0
[Hb.Fe3+]= 100
A100 - this is defined as the absorbance of hemoglobin imidazole complex when all hemoglobin is present in bound state.