Answer: The change in entropy that occurs in the system is 36.3 J/Kmol
Step-by-step explanation:
Given : 1 mole of isopropyl alcohol gives heat = 5.37 kJ
Thus 1.24 moles of isopropyl alcohol gives heat =
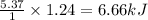
To calculate the temperature , we use the equation:
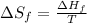
where,
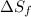 = Entropy of fusion = ?
= Entropy of fusion = ?
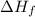 = enthalpy of fusion = 6.66 kJ/mol = 6660 J/mol (1kJ=1000J)
= enthalpy of fusion = 6.66 kJ/mol = 6660 J/mol (1kJ=1000J)
T = melting point =
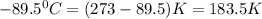
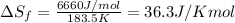
Thus the change in entropy that occurs in the system is 36.3 J/Kmol