Answer:
E°cell = - 1.3575 V
This reaction is spontaneous in the reverse direction
Step-by-step explanation:
The given cell reaction:
Cr³⁺(1.32 × 10⁻³ M) + Fe²⁺(aq) → Cr²⁺(aq) + Fe³⁺(1.14 M)
The given Gibbs free energy: ΔG = 131 kJ = 131 × 10³ J (∵ 1 kJ = 10³ J)
As we know,
ΔG = - n F E°cell
Here, n - the number of moles of electrons transferred = 1
F - Faraday constant = 96500
E°cell - cell potential = ?
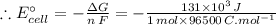
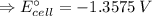
For a given chemical reaction if-
1. ΔG = negative and E°cell = positive
⇒ The reaction is spontaneous and proceeds spontaneously in the forward direction.
2. ΔG = positive and E°cell = negative
⇒ The reaction is non-spontaneous and proceeds spontaneously in the reverse direction.
Since, for this chemical reaction:
Cr³⁺(1.32 × 10⁻³ M) + Fe²⁺(aq) → Cr²⁺(aq) + Fe³⁺(1.14 M)
ΔG = + 131 × 10³ J ⇒ positive
and, E°cell = - 1.3575 V ⇒ negative
Therefore, this reaction is spontaneous in the reverse direction.