Answer:
Nitrification is a biological process by which ammonia is oxidized to nitrites (
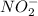 ) and then to nitrates (
) and then to nitrates (
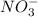 ) by action of specialized bacteria in nature (such as nitrosomonas and nitrobacter).
) by action of specialized bacteria in nature (such as nitrosomonas and nitrobacter).
Step-by-step explanation:
This two-step process utilizes molecular atmospheric oxygen and ammonia and ammonium components of soil and nature.
(a.) Ammonia and ammonium compounds to nitrite
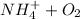 →
→
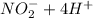
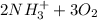 →
→
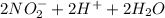
(b.) Nitrite to nitrate
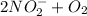 →
→
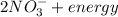
(c.) The reverse process involves reduction of nitrates to molecular nitrogen, called denitrification (with succinic acid in the equation below).
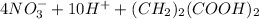 →
→
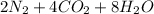
(
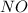 and
and
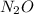 is an intermediate product in this process.)
is an intermediate product in this process.)