Answer:
The process is possible:
Step-by-step explanation:
We are going to find out if the entropy generated is greater than 0, if it is greater than 0, then the process is feasible. If it is not, the process is not feasible.
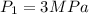
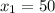
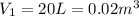
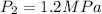
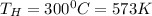
Received heat energy,
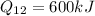
Work done,
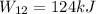
At state 1, using the steam table:
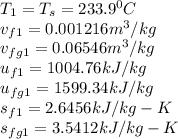
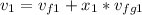
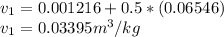
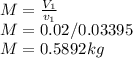
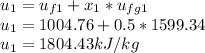
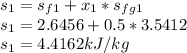
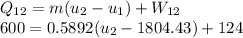
Solving for u₂
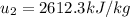
Since P₂ = 1.2 MPa, u₂ = 2612.2 kJ/kg,
then from steam table, T₂ = 200°C, S₂ = 6.5898 kJ/kg-K
The entropy generated will be:
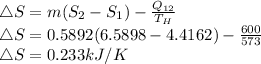
Since ΔS > 0, this process is possible