Answer:
Concentration of solution is 402g/mL
Step-by-step explanation:
The HCl solution has a purity of 35% w/w. That means there are 35g of HCl per 100g of solution. As density of solution is 1.15g/mL, volume of the solution is:
100g solution × (1mL / 1.15g) = 86.96mL. Thus concentration of solution in g/mL is:
35g of HCl / 86.96mL = 0.402g/mL. As 1L contains 1000mL:
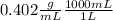 = 402g/mL
= 402g/mL
Concentration of solution is 402g/mL