Answer : 9773 kJ
Step-by-step explanation:
Exothermic reactions are defined as the reactions in which energy of the product is lesser than the energy of the reactants. The total energy is released in the form of heat and
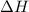 for the reaction comes out to be negative.
for the reaction comes out to be negative.
To calculate the moles :
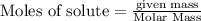

Given : Change in enthalpy on combustion of 1 mole of methanol = -1278 kJ
According to stoichiometry :
1 mole of methanol produce energy = 1278 kJ
Thus 7.647 moles of methanol produce energy =

Thus 9773 kJ of energy is produced from burning 245.0 g of methanol in excess oxygen