Answer: 406 hours
Step-by-step explanation:
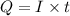
where Q= quantity of electricity in coloumbs
I = current in amperes = 39.5 A
t= time in seconds = ?
The deposition of copper at cathode is represented by:
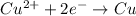
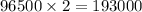 Coloumb of electricity deposits 1 mole of copper
Coloumb of electricity deposits 1 mole of copper
i.e. 63.5 g of copper is deposited by = 193000 Coloumb
Thus 19.0 kg or 19000 g of copper is deposited by =
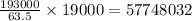 Coloumb
Coloumb
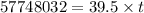
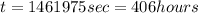 (1hour=3600s)
(1hour=3600s)
Thus it will take 406 hours to plate 19.0 kg of copper onto the cathode if the current passed through the cell is held constant at 39.5 A