Answer:
Ionic.
Step-by-step explanation:
Elements with higher electronegativity values are better at attracting electrons in a chemical bond.
- A chemical bond is considered "ionic" if the electronegativity difference between the two bonding atoms is greater than
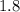 .
. - Otherwise, this chemical bond is considered "covalent".
In this example, the difference between the electronegativity of oxygen and lithium is
 . Since
. Since
 , the bond between the two elements would likely be ionic.
, the bond between the two elements would likely be ionic.
It is possible to reach the same conclusion based on the fact that lithium is a metal while oxygen is a nonmetal. When metal elements react with non-metal elements, the product is typically an ionic compound- with ionic bonds between the atoms.