Answer: The change of energy of the gas mixture during the reaction in 69 kJ
Step-by-step explanation:
According to first law of thermodynamics:
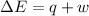
 =Change in internal energy
=Change in internal energy
q = heat absorbed or released
w = work done or by the system
w = work done on the system
w = -174 kJ
q = +243 kJ {Heat has increased so heat is absorbed}
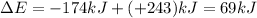
Thus the change of energy of the gas mixture during the reaction in kJ is 69