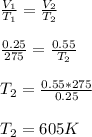Answer:
number of moles = 27.34 moles
the temperature of gas after it undergoes the isobaric expansion = 605 K
Step-by-step explanation:
Given that:
V = 0.25 m³
P = 250 kPa
T = 275 K
V₂ = 0.55 m³
P₂ = 760 kPa
a)
Using ideal gas equation ; PV = nRT
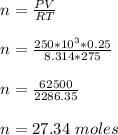
b) To calculate the temperature of gas after it undergoes the isobaric expansion; we have: