Answer: Yes interaction between an ammonia ligand and a metal cation is a lewis acid-base interaction
Step-by-step explanation:
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
As metal cation is short of electrons to complete its octet, thus it can easily accept an electron pair from ammonia which can easily donate its lone pair of electron and thus acts as lewis base.
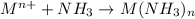
Thus interaction between an ammonia ligand and a metal cation is a lewis acid-base interaction