Answer: The actual free-energy change for the reaction -8.64 kJ/mol.
Step-by-step explanation:
The given reaction is as follows.
Fructose 1,6-bisphosphate
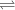 Glyceraldehyde 3-phosphate + DHAP
Glyceraldehyde 3-phosphate + DHAP
For the given reaction,
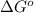 is 23.8 kJ/mol.
is 23.8 kJ/mol.
As we know that,
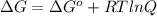
Here, R = 8.314 J/mol K, T =
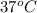
= (37 + 273) K
= 310.15 K
Fructose 1,6-bisphosphate =
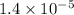 M
M
Glyceraldehyde 3-phosphate =
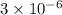 M
M
DHAP =
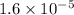 M
M
Expression for reaction quotient of this reaction is as follows.
Reaction quotient =
![\frac{[DHAP][\text{glyceraldehyde 3-phosphate}]}{[/text{Fructose 1,6-bisphosphate}]}](https://img.qammunity.org/2021/formulas/chemistry/college/x4irnk60teltcvqwwhw53yzxo4ijscgkws.png)
Q =
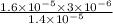
=
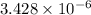
Now, we will calculate the value of
 as follows.
as follows.
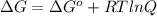
=
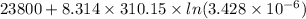
= -8647.73 J/mol
= -8.64 kJ/mol
Thus, we can conclude that the actual free-energy change for the reaction -8.64 kJ/mol.