Answer:
the mole fraction of A in solution is 0.32
Step-by-step explanation:
Let's consider a solution of two volatile liquids A and B
Vapor pressure of A is 385.0 torr
Vapor pressure of B is 104.0 torr
According to Dalton's Law
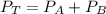 ---------- Equation (1)
---------- Equation (1)
where
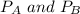 are partial pressure of A and B respectively.
are partial pressure of A and B respectively.
Using Roult's Law
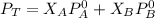 ---------- Equation (2)
---------- Equation (2)
where;
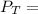 the total vapor pressure of the solution
the total vapor pressure of the solution
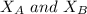 = mole fraction of A and B respectively
= mole fraction of A and B respectively
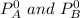 = vapor pressures of pure species of A and B
= vapor pressures of pure species of A and B
So;
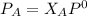
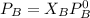
Replacing our given values for
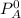 and
and
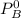 into equation (2) ; we have:
into equation (2) ; we have:
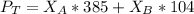
The sum of mole fractions is equal to 1
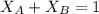
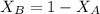
Hence;
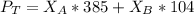
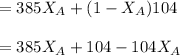
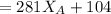 ------ Equation (3)
------ Equation (3)
It is given that mole fraction of vapor is equal to twice the mole fraction of liquid .
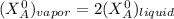
It is known that; a partial pressure of a component in gaseous phase is equal to mole fraction times the total pressures. As such;
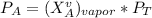
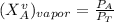
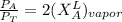 ------- Equation (4)
------- Equation (4)
Partial Pressure is given as follows :
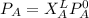
Thus; from equation 4
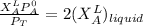
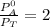
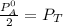
Replacing the above value into equation (8)
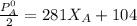
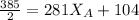
192.5 = 281
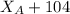
192.5 - 104 =
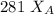
88.5 =
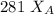
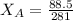
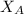 = 0.32
= 0.32
Thus, the mole fraction of A in solution is 0.32