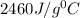Answer:
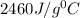
Step-by-step explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
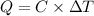
Q = Heat absorbed by calorimeter =
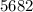 Joules
Joules
C = heat capacity of calorimeter = ?
Initial temperature of the calorimeter =
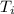 = 22.37°C
= 22.37°C
Final temperature of the calorimeter =
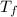 = 24.68°C
= 24.68°C
Change in temperature ,
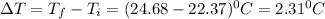
Putting in the values, we get:
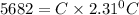
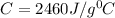
The heat capacity of the calorimeter is