Answer:
the value of the free energy change ΔG = 339.975 kJ
Step-by-step explanation:
The equation for the reaction is given as :
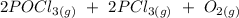
Equilibrium Constant K:
![K = ([PCl_3]^2[O_2])/([POCl_3]^2)\\\\K = ((0.0150)^2*(0.0100))/((10.00)^2)\\ \\K = 2.25*10^(-8)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/irza67xdzc3rh5ktctonrq2m3cwe51dqm9.png)
ΔGº = 489.75 kJ = 489750 J
T (temperature) = 750.0ºC = ( 750.0 + 273 )K
T (temperature) = 1023 K
R( rate constant) =
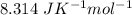
Using the equation :
ΔG = ΔG° + RT ㏑ K
ΔG = (489750) + ( 8.314)(1023)㏑ ( 2.25 × 10⁻⁸)
ΔG = 489750 + 8505.22 × (-17.6098)
ΔG = 339974.78 J
ΔG = 339.975 kJ
Thus, the value of the free energy change ΔG = 339.975 kJ