Answer:
The partial pressures of argon is 0.77 atm and krypton is 0.16 atm.
The total pressure exerted by the gaseous mixture is 0.93 atm
Step-by-step explanation:
Mass of argon gas =
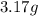
Mass of krypton gas =
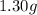
Mixture in terms of mole fractions:
Moles of argon gas =
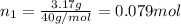
Moles of krypton gas =
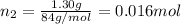
Mole fraction of argon gas =
=
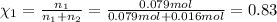
Mole fraction of carbon dioxide gas =
=
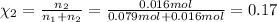
Pressure of the exerted by the gaseous mixture= P
Temperature of the mixture = T = 25.0 °C =25.0+273K=298.0 K
Volume of the container in which mixture is kept =
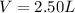
Moles of gases,n =
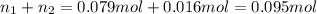
PV=nRT
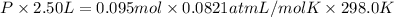
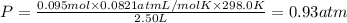
Partial pressure of argon gas =
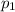
Partial pressure of krypton gas =
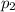
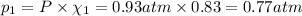
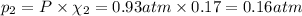
The partial pressures of argon is 0.77 atm and krypton is 0.16 atm.
The total pressure exerted by the gaseous mixture is 0.93 atm