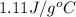Answer : The specific heat of the metal is,
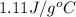
Explanation
In this problem we assumed that heat given by the hot body is equal to the heat taken by the cold body.
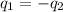
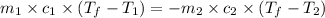
where,
 = specific heat of metal = ?
= specific heat of metal = ?
 = specific heat of water =
= specific heat of water =
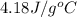
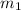 = mass of metal = 95.0g
= mass of metal = 95.0g
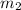 = mass of water = 50.0 g
= mass of water = 50.0 g
 = final temperature of mixture =
= final temperature of mixture =
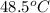
 = initial temperature of metal =
= initial temperature of metal =
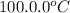
 = initial temperature of water =
= initial temperature of water =
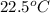
Now put all the given values in the above formula, we get
![(95.0g)* c_1* (48.5-100.0)^oC=-[(50.0g)* 4.18J/g^oC* (48.5-22.5)^oC]](https://img.qammunity.org/2021/formulas/chemistry/high-school/a8vi1sf0qw8aqa6ppmcn8rmq9njl9o7l6a.png)
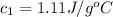
Therefore, the specific heat of the metal is,