Answer:
1.05 × 10⁻⁸ N
Step-by-step explanation:
Using Coulomb's law
F force of attraction between them = k
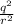 = k
= k
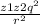
K coulomb constant = 8.99 × 10⁹ N m²C⁻²
Z₁ = + 2 ( valency electron)
Z₂ = -1 ( valency electron)
r is the distance between then since they just touch one another = 0.085 nm + 0.125 nm = 0.21 nm = 0.21 × 10⁻⁹ m
q charge = 1.602 × 10⁻¹⁹ C of an electron
F = ( 2 × 1 × 8.99 × 10⁹ N m²C⁻² × (1.602 × 10⁻¹⁹ C)² / ( 0.21 × 10⁻⁹ m )² = 1046.34 × 10 ⁻¹¹ = 1.05 × 10⁻⁸ N