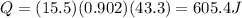Answer:
605.4 J
Step-by-step explanation:
When a certain substance absorbs a certain amount of energy, its temperature increases according to the equation:
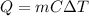
where
Q is the heat absorbed
m is the mass of the substance
C is the specific heat capacity of the substance
 is the change in temperature
is the change in temperature
In this problem, we have:
m = 15.5 g is the mass of the piece of aluminium
C = 0.902 J/g⁰C is the specific heat of the aluminium
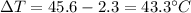 is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)
is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)
Therefore, the energy absorbed is