Answer:
5.93° C
Step-by-step explanation:
We can solve this problem by using Charles law. It states that when the pressure of a gas is constant, the ratio of volume and temperature of the gas is constant. Mathematically:
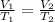
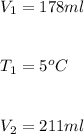
Hence, the final temperature,
 , will be:
, will be:
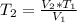
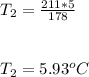
The final temperature of the gas is 5.93°C