Answer:
Methanoic acid or formic acid:
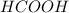 .
.
Step-by-step explanation:
Hello,
In this case, when methanol reacts with a strong oxidizing agent as potassium permanganate, the first oxidation product is methanal (formaldehyde) as shown below:
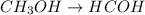
Afterwards, the methanal continues reacting with the potassium permanganate to finally yield methanoic acid (formic acid):
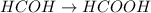
This is understood by the fact that oxidation in organic chemistry is also related with the increase of oxygen atoms in the oxidized molecule.
Best regards.
Best regards.