Answer: 3)
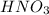 and
and

Step-by-step explanation:
According to the Arrhenius concept, an acid is a substance that ionizes in the water to give hydronium ion or hydrogen ion.
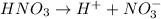
According to the Arrhenius concept, a base is a substance that ionizes in the water to give hydroxide ion.
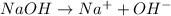
Thus
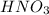 is an arrhenius acid and
is an arrhenius acid and
 is an arrhenius base.
is an arrhenius base.