Answer:
Incomplete question: The concentration of the aqueous solution is 0.65 mM
The percentage of dissociation is 19.23%
Step-by-step explanation:
The pKa of the 4-chlorobutanoic acid is 4.53, thus:
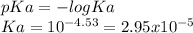
The initial concentration is 0.65 mM = 6.5x10⁻⁴M
The reaction is:
HA + H₂O = H₃O⁺ + A⁻
I 6.5x10⁻⁴ 0 0
C -x +x +x
E 6.5x10⁻⁴ -x x x
The Ka is:
![Ka=([H_(3)O^(+) ][A^(-)] )/([HA]) \\2.95x10^(-5) =(x*x)/(6.5x10^(-4)-x ) \\x^(2) +2.95x10^(-5)x-1.92x10^(-8) =0\\x=1.25x10^(-4) M=[A^(-) ]](https://img.qammunity.org/2021/formulas/chemistry/college/u8tzz23tu79wvaarh42yym50s6vrg01hi2.png)
The percentage of dissociated is:
![P=([A^(-)] )/([HA]) *100=(1.25x10^(-4) )/(6.5x10^(-4) ) *100=19.23](https://img.qammunity.org/2021/formulas/chemistry/college/xbjewl6mpmw1duj27ucmmnx1rnhpm8qjkz.png) %
%