Answer:
a. Molarity=
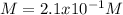
b. Molality=
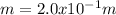
Step-by-step explanation:
Hello,
In this case, given the information about the aniline, whose molar mass is 93g/mol, one could assume the volume of the solution is just 200 mL (0.200 L) as no volume change is observed when mixing, therefore, the molarity results:
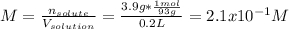
Moreover, the molality:
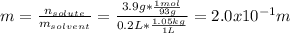
Best regards.