The given question is incomplete. The complete question is ;
A student dissolves of 15 g aniline in 200 ml of a solvent with a density of 1.05 g/ml. The student notices that the volume of the solvent does not change when the aniline dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits.
Answer: The molarity is 0.81 M and molality is 0.82 m
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
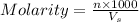
where,
n= moles of solute
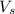 = volume of solution in ml = 200 ml
= volume of solution in ml = 200 ml
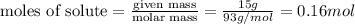
Now put all the given values in the formula of molarity, we get
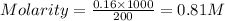
Thus molarity is 0.81 M
Molality of a solution is defined as the number of moles of solute dissolved per kg of the solvent.
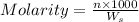
where,
n = moles of solute
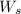 = weight of solvent in g
= weight of solvent in g
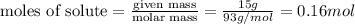
Mass of solution =
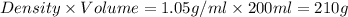
mass of solvent = mass of solution - mass of solute = (210 - 15) g = 195 g
Now put all the given values in the formula of molality, we get
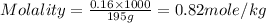
Therefore, the molality of solution is 0.82m