Answer:
a.
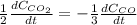
b.
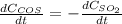
c.
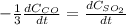
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
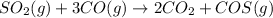
For which rates of consumption are related as follows, taking into account the change in the concentration with respect to the time and each species stoichiometric coefficient:
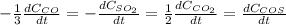
For the given requirements, each rate of formation turns out as shown below:
a.
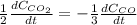
b.
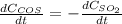
c.
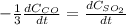
Best regards.