The new volume will be 32.88 litres if the pressure increases to 260 kPa.
Step-by-step explanation:
Data given:
Initial volume of the gas = 45 ml
initial pressure of the gas = 190 KPa
final pressure on the gas = 260 kpa
final volume =?
from the data provided in the question it is assumed that Boyle's law is used to know the new volume of the gas.
so,
P1V1 = P2V2
Rearranging the equation to determine the final volume of the gas:
V2 =
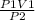
V2 =
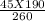
= 32.88 Litres
the new volume will be 32.88 litres when pressure is increased to 260 Kpa.