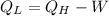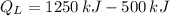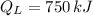Answer:
a) This heat engine is possible since real efficiency is lesser than theoretical efficiency, b)
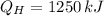 , c)
, c)
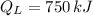 .
.
Step-by-step explanation:
a) The maximum theoretical efficiency of a heat efficiency is given by the Carnot's cycle, whose formula is:
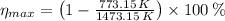
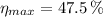
This heat engine is possible since real efficiency is lesser than theoretical efficiency.
b) The heat supplied to the engine by the heat source:
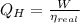
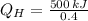
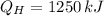
c) The heat rejected to heat sink is: