Answer:
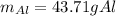
Step-by-step explanation:
Hello,
In this case, since the heat lost by the aluminium is gained by the water, the following equality is achieved:
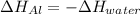
Which in terms of masses, heat capacities and temperatures is:
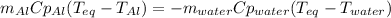
Thus, by solving for the mass of aluminium, we obtain:
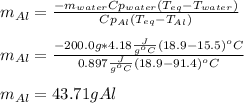
Best regards.