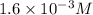Answer: The molarity of this second dilution is
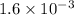
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
According to the neutralization law,

where,
 = molarity of stock solution = 0.20 M
= molarity of stock solution = 0.20 M
 = volume of stock solution = 5.00 ml
= volume of stock solution = 5.00 ml
 = molarity of dilute solution = ?
= molarity of dilute solution = ?
 = volume of dilute solution = 25.00 ml
= volume of dilute solution = 25.00 ml
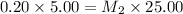

The molarity of this first dilution solution is 0.04 M

where,
 = molarity of stock solution = 0.04 M
= molarity of stock solution = 0.04 M
 = volume of stock solution = 2.00 ml
= volume of stock solution = 2.00 ml
 = molarity of dilute solution = ?
= molarity of dilute solution = ?
 = volume of dilute solution = 50.00 ml
= volume of dilute solution = 50.00 ml
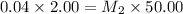
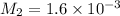
The molarity of this second dilution is