Answer:
–187.9 J/K
Step-by-step explanation:
The equation that relates the three quantities is:
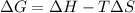
where
 is the Gibbs free energy
is the Gibbs free energy
 is the change in enthalpy of the reaction
is the change in enthalpy of the reaction
T is the absolute temperature
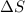 is the change in entropy
is the change in entropy
In this reaction we have:
ΔS = –187.9 J/K
ΔH = –198.4 kJ = -198,400 J
T = 297.0 K
So the Gibbs free energy is
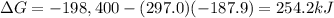
However, here we are asked to say what is the entropy of the reaction, which is therefore
ΔS = –187.9 J/K