Answer:
1. E=5320.3J
2. E= 1773.45J
3. E=6.76*10^{-21}J
4. E=2.25*10^{-21}J
Step-by-step explanation:
1. the thermal energy is given by the formula
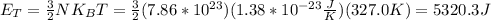
where KB is the Boltzmann's constant, T is the temperature and N is the number of molecules in the system.
2. Each degree of freedom contains one half of the total energy. Hence, the energy for one degree of freedom is
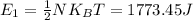
3.
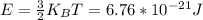
4.
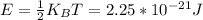
Hope this helps!!