Answer:
A
Step-by-step explanation:
We can use the heat transfer equation:
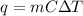
Therefore:
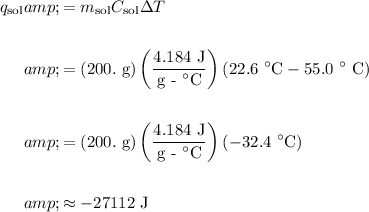
In conclusion, the answer is A.
Note: Assuming that the reaction took place in the water, the heat released by the reaction should be positive instead of negative. Because the temperature of the water decreased, the reaction is endothermic. Hence, the water lost heat (what we calculated above) while the reaction absorbed heat. The heat released (or, rather, absorbed) by the reaction is thus +27,112 J. However, for the purposes of this question, A is the best choice.