Answer: The actual yield of
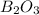 is 60.0 g
is 60.0 g
Explanation:-
The balanced chemical reaction :
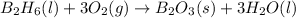
Mass of
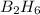 =
=
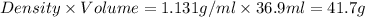
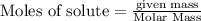
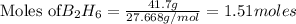
According to stoichiometry:
1 mole of
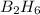 gives = 1 mole of
gives = 1 mole of
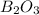
1.51 moles of
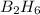 gives =
gives =
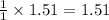 moles of
moles of
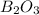
Theoretical yield of
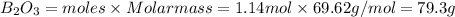
Percent yield of
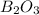 =
=
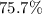
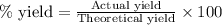
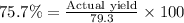
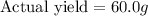
Thus the actual yield of
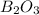 is 60.0 g
is 60.0 g