When determining the equilibrium concentrations for the reaction
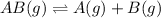
;
![K_c = ([A][B])/([AB])](https://img.qammunity.org/2021/formulas/chemistry/high-school/sssojx1a43sgcbw40ao14xds7g1qicwo.png)
a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation.
![K_c = (x^2)/([AB]-x) \approx (x^2)/([AB])](https://img.qammunity.org/2021/formulas/chemistry/high-school/mqq1o3ng9419npidzzzwh06xeoq6q8ar.png)
Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required.Use simplifying assumptions - Quadratic formula required 1. [AB] = 0.0178 M;
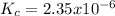
2. [AB] = 0.00204 M;
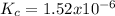
3. [AB] =0.451 M;

= 0.000905 4. [AB] = 0.0174 M;

= 0.0000925 5. [AB] = 0.396 M;

= 0.00228