Answer: Option (e) is the correct answer.
Step-by-step explanation:
Equilibrium is defined as the state of a chemical reaction in which the rate of forward reaction is equal to the rate of backward reaction.
The state of equilibrium is represented by a double sided arrow as "
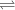 " in a chemical equation.
" in a chemical equation.
For example,
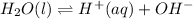
Therefore, we can conclude that when a reaction system is at equilibrium then the rates of the reaction in the forward and reverse directions are exactly equal.