Answer:
Please read the answer below
Step-by-step explanation:
The energies in a infinite square-well potential of width a is given by
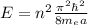
where me=9.1*10^{-31}kg, hbar=1.05*10^{-34}Js and a= 0.2*10^{-9}m.
From the state n=4 the electron can pass to state n=3, n=2 and n=1. The different transitions can be
n4->n3=E4-E3
n3->n2=E3-E2
n2->n1=E2-E1
Hence, by replacing we have that the photon energies emitted are given by
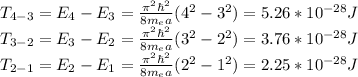
However, the transitions T4-2, T4-3, T3-1 are also allowed
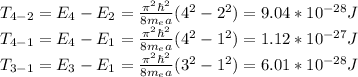
hope this helps!!