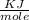Answer:
The Enthalpy of the reaction ΔH = 0.945
Step-by-step explanation:
Given data
Mass = 0.15 kg
Change in temperature = 24 - 21 = 3 °c
Enthalpy of the solution is given by
ΔH = m
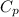 ΔT
ΔT
ΔH = 0.15 × 4.2 × 3
ΔH = 1.89 KJ
Enthalpy change per mole
ΔH =
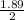
ΔH = 0.945
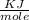
Therefore the Enthalpy of the reaction ΔH = 0.945