Answer:
The value of the heat capacity of the Calorimeter
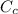 = 54.4
= 54.4
Step-by-step explanation:
Given data
Heat added Q = 4.168 KJ = 4168 J
Mass of water
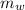 = 75.40 gm
= 75.40 gm
Temperature change = ΔT = 35.82 - 24.58 = 11.24 ° c
From the given condition
Q =
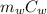 ΔT +
ΔT +
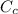 ΔT
ΔT
Put all the values in above equation we get
4168 = 75.70 × 4.18 × 11.24 +
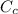 × 11.24
× 11.24
611.37 =
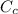 × 11.24
× 11.24
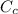 = 54.4
= 54.4
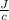
This is the value of the heat capacity of the Calorimeter.