The given question is incomplete. The complete question is :
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reaction is endothermic. What do you expect to happen to the concentration of NO if the temperature is doubled? 2NOBr(g) ⇌ 2NO(g) + Br2(g)
A) The concentration of NO will increase.
B) The concentration of NO will decrease.
C) There will be no change in [NO].
D) A catalyst will be needed to make a change in concentration.
E) The change in concentration of [NO] will depend on the size of the vessel.
Answer: The concentration of NO will increase.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
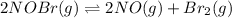
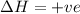
If the temperature is doubled, according to the Le-Chatelier's principle, the equilibrium will shift in the direction where decrease in temperature is taking place. As the forward reaction is endothermic, the equilibrium will shift in the right direction. i.e. towards products.
Thus the concentration of NO will increase.