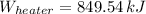Answer:
Step-by-step explanation:
The piston-cylinder system is modelled after the First Law of Thermodynamics:

The electrical energy supplied by the resistance heater is:
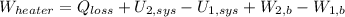
Let suppose that air behaves ideally, so that:
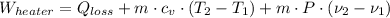
The initial specific volume is determined by the use of the equation of state for ideal gases:
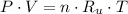
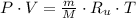
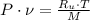
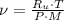
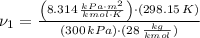
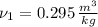
The final specific volume can be derived from the following relationship:
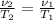
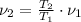
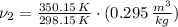
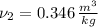
The energy supply is:
![W_(heater) = 60\,kJ + (15\,kg)\cdot \left[\left(0.718\,(kJ)/(kg\cdot ^(\textdegree)C) \right)\cdot (77\,^(\textdegree)C- 25\,^(\textdegree)C) + (300\,kPa)\cdot \left(0.346\,(m^(3))/(kg)-0.295\,(m^(3))/(kg) \right)\right]](https://img.qammunity.org/2021/formulas/engineering/college/1kxtnemfn2fc4qwcsxvv09mtdtxfkv2m77.png)